Purpose of This Guideline
Date of current publication: July 19, 2021
Lead author: Ethan Cowan, MD, MS
Writing group: Joseph P. McGowan, MD, FACP, FIDSA; Steven M. Fine, MD, PhD; Rona Vail, MD; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 24, 2018
This guideline on diagnosis and management of acute HIV infection was developed by the Medical Care Criteria Committee of New York State Department of Health AIDS Institute (NYSDOH AI) to guide clinicians in NYS who provide ambulatory, inpatient, and emergency medical care for adults ≥18 years old who present with signs or symptoms of acute HIV infection or report an exposure within the past 4 weeks.
This guideline provides evidence-based clinical recommendations for the diagnosis and treatment of acute HIV infection in adults, with the goals of ensuring that NYS clinicians are able to:
- Recognize the risks for and signs and symptoms of acute HIV, include HIV infection in the differential diagnosis, and consider HIV testing in any person who presents with signs and symptoms suggestive of influenza (“flu”), mononucleosis (“mono”), or other viral syndromes, including suspected COVID-19.
- Perform appropriate diagnostic and confirmatory testing when HIV infection is suspected and manage the treatment of acute HIV.
- Meet the NYS requirements for reporting and partner notification.
- Recommend or offer immediate initiation of antiretroviral therapy (ART) to improve the patient’s health and reduce the risk of HIV transmission; refer and confirm that patients can access optimal HIV care.
- Initiate or refer the patient for prevention services.
| TERMINOLOGY |
|
Early diagnosis for early treatment: Accumulating evidence supports a decision to begin HIV treatment at the time of diagnosis Lundgren, et al. 2015. Initiation of ART during acute infection may have several beneficial clinical outcomes, including improved preservation of immunologic function, significantly reduced time to viral suppression, and reduction of the viral reservoir, which could be important for cure strategies Pires, et al. 2004; Streeck, et al. 2006; Koegl, et al. 2009; Hocqueloux, et al. 2010; Ananworanich, et al. 2012; Buzon, et al. 2012; Lafeuillade, et al. 2012; Margolick, et al. 2015; Phanuphak, et al. 2015; Le, et al. 2013; Saez-Cirion, et al. 2013; Pilcher, et al. 2017. The risk of sexual transmission of HIV during acute or recent infection is significantly higher than during chronic infection Pilcher, et al. 2004; Hollingsworth, et al. 2008; Pinkerton 2008; Hollingsworth, et al. 2015; this difference likely correlates with high levels of viremia and is consistent with other routes of transmission Bellan, et al. 2015. The public health benefit of early ART initiation is well documented, with a significant reduction of HIV transmission among virally suppressed individuals. Further, in September 2017, the NYSDOH endorsed the consensus from the Prevention Access Campaign that undetectable = untransmittable (“U = U”), which indicates that individuals with a durable (≥6 months) undetectable viral load will not sexually transmit HIV NYSDOH 2017; Prevention Access Campaign 2018.
Recognizing and diagnosing acute HIV infection is crucial to linking patients to care early and presents an important opportunity to reduce HIV transmission. Factors that may contribute to the increased risk for transmission during acute infection include:
- Hyperinfectivity associated with both markedly high viral load levels (often much greater than 10 million viral copies/mm3) and increased infectiousness of the virus Quinn, et al. 2000; Ma, et al. 2009.
- Missed HIV diagnosis Chin, et al. 2013; Nakao, et al. 2014. Because the nonspecific flu- or mono-like symptoms are frequently unrecognized as symptoms of acute HIV infection or attributed to a nonspecific viral syndrome, the diagnosis is often missed. Missed diagnosis of acute HIV infection results in a lost opportunity to recommend treatment and risk-reduction counseling that could reduce both viral load levels and high-risk behavior Colfax, et al. 2002; Steward, et al. 2009; Fonner, et al. 2012; Kroon, et al. 2017; Rutstein, et al. 2017.
For many reasons, detecting acute HIV infection is an essential link in the chain of prevention. Evidence demonstrates that patients with a recent diagnosis of HIV are more likely to reduce risk behaviors if they are given counseling at the time of testing Steward, et al. 2009; Fonner, et al. 2012 and are linked to primary HIV care Metsch, et al. 2008. In addition, for those who elect to initiate ART, their risk of transmission is significantly diminished Cohen, et al. 2011; Cohen, et al. 2016.
| KEY POINTS |
|
| New York State Law |
|
Note on “experienced” and “expert” HIV care providers: Throughout this guideline, when reference is made to “experienced HIV care provider” or “expert HIV care provider,” those terms are referring to the following 2017 NYSDOH AI definitions:
- Experienced HIV care provider: Practitioners who have been accorded HIV Experienced Provider status by the American Academy of HIV Medicine or have met the HIV Medicine Association’s definition of an experienced provider are eligible for designation as an HIV Experienced Provider in New York State. Nurse practitioners and licensed midwives who provide clinical care to individuals with HIV in collaboration with a physician may be considered HIV Experienced Providers as long as all other practice agreements are met (8 NYCRR 79-5:1; 10 NYCRR 85.36; 8 NYCRR 139-6900). Physician assistants who provide clinical care to individuals with HIV under the supervision of an HIV Specialist physician may also be considered HIV Experienced Providers (10 NYCRR 94.2)
- Expert HIV care provider: A provider with extensive experience in the management of complex patients with HIV.
Presentation and Diagnosis
| RECOMMENDATIONS |
New York State HIV Testing Requirements
Presentation
When Acute HIV Infection Is Suspected
Diagnosis
ART Initiation
Partner Notification
|
Abbreviations: Ab, antibody; Ag, antigen; ART, antiretroviral therapy; NAT, nucleic acid test; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection. |
The time from HIV infection to detection of the virus depends on the test that is used. Figure 1, below, illustrates the window of detection of HIV infection according to Ab, Ag/Ab combination, and HIV RNA tests.
Figure 1: HIV Test Window of Detection [a,b]
![Figure 1: HIV Test Window of Detection [a,b]](https://www.hivguidelines.org/wp-content/uploads/2023/07/NYSDOH-AI-HIV-Testing-Figure-1_7-20-2023_HG.jpg)
Abbreviations: IgG, immunoglobulin G; IgM, immunoglobulin M; NAT, nucleic acid test.
Notes:
- Figure reproduced from CDC: HIV Diagnostic Tests.
- Without PrEP or PEP exposure; PrEP or PEP exposure may delay seroconversion. Very early treatment of acute HIV infection may also alter the serologic response Stekler, et al. 2023; Hare, et al. 2006; Kassutto, et al. 2005.
Download figure: HIV Test Window of Detection
Presentation
Patients acutely infected with HIV will often experience at least some symptoms of acute retroviral syndrome (ARS). Fever and influenza- or mononucleosis-like symptoms are common in acute HIV infection but are nonspecific. Rash, mucocutaneous ulcers, oropharyngeal candidiasis, and meningismus are more specific and should raise the index of suspicion (see below for a more extensive list of signs and symptoms). The mean time from exposure to onset of symptoms is generally 2 to 4 weeks, with a range of 5 to 29 days; however, some cases have presented with symptoms up to 3 months after exposure Apoola, et al. 2002. Theoretically, this time course may be prolonged in patients who become infected while on PEP or PrEP.
| Box 1: Acute Retroviral Syndrome |
Signs and symptoms of ARS with the expected frequency among symptomatic patients are listed below [a]. The most specific symptoms in this study were oral ulcers and weight loss; the best predictors were fever and rash. The index of suspicion should be high when these symptoms are present.
|
|
Note:
|
Diagnosis
Acute HIV infection is often not recognized in the primary care setting because the symptom profile is similar to that of influenza, mononucleosis, and other common illnesses. Furthermore, patients often do not recognize that they may have recently been exposed to HIV. Therefore, the clinician should have a high index of suspicion for acute HIV infection in a patient who may have recently engaged in behavior involving sexual or parenteral exposure to another individual’s blood or body fluids and who is presenting with a febrile, influenza-, or mononucleosis-like illness. Identifying acute HIV infection during pregnancy is particularly important because effective intervention can prevent mother-to-child transmission Patterson, et al. 2007.
High levels of HIV RNA detected in plasma through sensitive NAT, combined with a negative or indeterminate HIV screening or type-differentiation test, support the presumptive diagnosis of acute HIV infection Robb, et al. 2016; DHHS 2019.
When low-level viremia is reported by HIV RNA testing (<5,000 copies/mL) in the absence of serologic confirmation of HIV infection, HIV RNA testing should be repeated to exclude a false-positive result Hecht, et al. 2002. Repeat HIV RNA testing with a result that indicates the presence of low-level viremia may represent true HIV infection, warranting appropriate counseling regarding transmission risk and initiation of ART.
HIV RNA levels tend to be very high in acute infection; however, a low value may represent any point on the upward or downward slope of the viremia associated with acute infection or could simply represent chronic infection. HIV RNA can also be suppressed during acute infection in patients who are taking PrEP. Plasma HIV RNA levels during acute infection do not appear significantly different in patients who are and are not symptomatic Patterson, et al. 2007. Viremia occurs approximately 1 to 2 weeks before the detection of a specific immune response. Patients diagnosed with acute infection by HIV RNA testing should always receive follow-up diagnostic testing 3 weeks later to confirm infection (see the standard HIV laboratory testing algorithm). Figure 2, below, illustrates diagnostic testing for acute HIV infection.
| KEY POINTS |
|
Figure 2: Diagnostic Testing for Acute HIV Infection
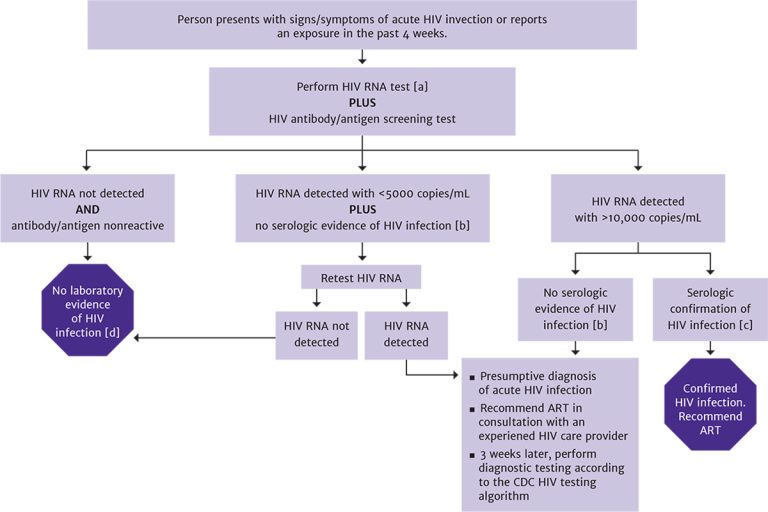
Notes:
- Viremia will be present several days before antibody detection.
- The absence of serologic evidence of HIV infection is defined as nonreactive screening result (antibody or antibody/antigen combination) or a reactive screening result with a nonreactive or indeterminate antibody-differentiation confirmatory result.
- Serologic confirmation as defined by the CDC HIV testing algorithm. Western blot is no longer recommended as the confirmatory test because it may yield an indeterminate result during the early stages of seroconversion and may delay confirmation of diagnosis.
- No further testing is indicated.
Management, Including While on PEP or PrEP
| RECOMMENDATIONS |
Managing Acute HIV
Initiating ART
|
Abbreviations: ART, antiretroviral therapy; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis. |
Patients are at greatest risk for transmitting HIV during periods of high viremia early in infection. Clinicians should counsel patients with acute HIV about the increased risk of transmission during the 6 months after infection. Partner notification Golden, et al. 2004, counseling on safer sex, and screening for other sexually transmitted infections are all essential in the management of any new HIV diagnosis.
Consultation: When choosing an ART regimen for a patient with acute HIV infection, clinicians should consult a care provider experienced in treating acute HIV infection.
- Data are insufficient to support ART regimens for treatment of acute HIV infection specifically; instead, the choice of regimen should be made based on recommendations for selecting an initial ART regimen.
- The risks of transmitted resistance should be considered when prescribing ART while awaiting HIV resistance results.
- The risks of acquired mutations should be considered in those who acquire HIV while on PrEP.
Clinicians who do not have access to experienced HIV care providers should call the CEI Line at 866-637-2342.
Benefits and Risks of ART
This section is an excerpt from the NYSDOH AI guideline Rapid ART Initiation
| RECOMMENDATIONS |
Benefits and Risks of ART
|
ART is the use of pharmacologic agents that have specific inhibitory effects on HIV replication. These agents belong to distinct classes of drugs with different mechanisms of action.
A list of all commercially available antiretroviral (ARV) drugs that are approved by the U.S. Food and Drug Administration for the treatment of HIV/AIDS is available here.
Benefits of ART
ART has led to dramatic reductions in HIV-associated morbidity and mortality CDC(a) 2022. In resource-rich settings, life expectancy of patients with HIV infection with access to early ART is approaching that of the general population Xia, et al. 2022; Siddiqi, et al. 2016. A number of randomized clinical trials have demonstrated the benefits of ART in reducing HIV-related morbidity and mortality, irrespective of the degree of immune suppression at treatment initiation Lundgren, et al. 2015; Severe, et al. 2010. Thus, ART should be recommended to all individuals with HIV infection.
With proper selection of an initial regimen (see the NYSDOH AI guideline Selecting an Initial ART Regimen) and good patient adherence, durable virologic suppression (i.e., lifetime control of viral load) is achieved in virtually all patients with HIV. Virologic suppression almost invariably leads to immunologic recovery, followed by reductions in the incidence of opportunistic infections and malignancies.
The measurable goals of treatment include:
- Viral suppression as measured by an HIV-1 RNA level below the limits of detection
- Immune reconstitution as measured by an increase in or maintenance of CD4 cell count
- Reduction in HIV-associated complications, including AIDS-related and non-AIDS-related conditions
ART also reduces morbidity and mortality from causes not related to HIV. In a randomized study comparing continuous ART with CD4-guided treatment interruption, a mortality benefit was observed in participants on continuous ART El-Sadr, et al. 2006. This benefit was attributed to a reduction in deaths from cardiovascular, renal, and hepatic causes. ART decreases the inflammatory milieu associated with ongoing HIV replication. It is postulated that ART-mediated reductions in proinflammatory cytokines lead to lower rates of clinical complications associated with the proinflammatory state Hileman and Funderburg 2017.
Reduced HIV transmission: ART for people with HIV is now part of the established strategy aimed at reducing HIV transmission and is an essential component of prevention interventions along with risk-reduction counseling, safer-sex practices, avoidance of needle-sharing, and HIV pre-exposure and post-exposure prophylaxis (PrEP and PEP; see the NYSDOH AI guidelines PrEP to Prevent HIV and Promote Sexual Health and PEP to Prevent HIV Infection). Antiretroviral treatment as prevention is associated with greater reductions in HIV transmission than any preventative modality studied to date. In HPTN 052, a large randomized clinical trial of HIV-serodifferent couples, early treatment of the partner with HIV was associated with a 96% reduction in HIV transmission compared with a delayed treatment approach Cohen, et al. 2011. In long-term follow-up of study participants, linked transmissions between partners were found to occur only when the index partner was viremic Cohen, et al. 2016. In observational studies, including the Opposites Attract, PARTNER, and PARTNER2 studies, no phylogenetically linked HIV transmission was observed in serodifferent couples in which the index partner was virologically suppressed on ART Rodger, et al. 2019; Bavinton, et al. 2018; Rodger, et al. 2016. The evidence thus suggests that the risk of sexual transmission of HIV during virologic suppression is negligible. ART should be recommended to all patients with HIV infection to prevent transmission to sex partners and, by extrapolation, to needle-sharing partners. Despite its potent benefit in reducing HIV transmission, ART does not obviate the use of condoms or clean syringes. Those harm reduction measures, along with the use of HIV PrEP for partners who do not have HIV infection, will help reduce the incidence of other sexually transmitted infections and viral hepatitis and should be integrated into patient counseling at ART initiation.
Reduced perinatal HIV transmission: Studies have shown that the administration of ART during pregnancy or intrapartum significantly reduces the risk of perinatal HIV transmission Cohen, et al. 2011; Guay, et al. 1999; Connor, et al. 1994, adding to the body of evidence that lower viral load reduces transmission risk.
Reduced complications: Accumulating evidence suggests that early initiation of ART or reduced cumulative time with detectable plasma viremia is associated with reductions in the likelihood of certain complications, such as cardiovascular disease, neurocognitive dysfunction, severe bacterial infections, and some non-HIV-related malignancies, and delayed initiation of ART is associated with long-term disparities in clinical outcomes Lundgren, et al. 2023; O'Connor, et al. 2017; Ho, et al. 2012; Sigel, et al. 2012; Winston, et al. 2012; Ellis, et al. 2011; Garvey, et al. 2011; Silverberg, et al. 2011; Ho, et al. 2010; Lichtenstein, et al. 2010; Bruyand, et al. 2009; Guiguet, et al. 2009; Marin, et al. 2009; Tozzi, et al. 2007; El-Sadr, et al. 2006. Cohort data also demonstrate that although older patients are more likely than younger patients to achieve virologic suppression, they are less likely to achieve an immunologic response, as measured by an increase of CD4 count by 100 cells/mm3, and that patients ≥55 years old may be at higher clinical risk even after starting ART Sabin, et al. 2008. The poor immunologic recovery seen in older patients is associated with higher morbidity and mortality, particularly cardiovascular events van Lelyveld, et al. 2012. In one study, men ≥50 years old with CD4 counts of 351 to 500 cells/mm3 who initiated ART were able to achieve similar immunologic responses as younger men who initiated at lower CD4 cell counts Li, et al. 2011.
Risks of ART
Despite the excellent tolerability of contemporary ART regimens, adverse effects, long-term drug toxicities, and drug-drug interactions continue to pose some relative or limited risk, which necessitates patient counseling about the potential for ART-associated adverse events in the short and long term. These risks include tolerability issues, which may affect quality of life, and possible long-term toxicities—primarily a low relative risk of renal and cardiovascular disorders or decreased bone density of uncertain clinical significance Hoy, et al. 2017; Monteiro, et al. 2014; Friis-Moller, et al. 2010. Excess weight gain has been observed in patients receiving regimens containing integrase strand transfer inhibitors (e.g., dolutegravir and bictegravir) and/or tenofovir alafenamide but the clinical significance is unknown, and investigation is needed Palella, et al. 2023; Verburgh, et al. 2022; Bourgi(a), et al. 2020; Bourgi(b), et al. 2020. Renal and bone density issues are largely eliminated with newer formulations of ARV medications. Fatal drug reactions from ART are exceedingly rare.
Many ARV combinations are now available in single-pill, fixed-dose combination formulations. Thus, the pill burden associated with early ART regimens has been largely eliminated. Nevertheless, lifelong adherence to medications may constitute a challenge to some, particularly when treatment with a single daily tablet is not feasible.
Compared with early ARV combinations, current preferred ART regimens (see the NYSDOH AI guideline Selecting an Initial ART Regimen) are associated with higher rates of durable virologic suppression. Lack of virologic suppression in a patient on ART should prompt the clinician to evaluate patient adherence and provide intensive support to those reporting challenges in this domain. Failure to achieve and maintain virologic suppression may lead to the emergence of resistance-associated mutations (RAMs). A large cohort study demonstrated that virologic failure with contemporary ART regimens is associated with the infrequent emergence of RAMs Scherrer, et al. 2016. Nevertheless, RAMs can emerge with current first-line therapies. Resistance to ARV medications may compromise the potential for long-term virologic suppression, simple dosing schedules, and the tolerability of future treatment options.
ART initiation is associated with a risk of immune reconstitution inflammatory syndrome (IRIS). IRIS is a clinical syndrome characterized by new or worsening infectious and non-infectious complications observed after the initiation of ART (see the NYSDOH AI guideline Management of IRIS). The risk of IRIS increases when ART is begun at low CD4 cell counts (<100 cells/mm3) or with the presence of specific opportunistic infections Manabe, et al. 2007. Although the risk of IRIS is not a contraindication to initiating ART, clinicians and patients should be aware that the risk of developing IRIS is increased among individuals with low CD4 cell counts. Patients at increased risk should be informed of the potential for a paradoxical clinical worsening after ART initiation.
Risks of Untreated HIV
Results from the START trial Lundgren, et al. 2015 and strong cohort data show that untreated HIV infection leads to increased morbidity and mortality from both HIV-related and non-HIV-related conditions, even at high CD4 cell counts. Together with the dramatic reduction in HIV transmission risk with effective treatment, these data support initiating ART regardless of CD4 cell count, including in patients diagnosed with acute HIV infection (see the NYSDOH AI guideline Diagnosis and Management of Acute HIV Infection). Patients in care who are documented long-term nonprogressors or elite controllers are a group that may warrant special consideration (see NYSDOH AI guideline Rapid ART Initiation > Special Considerations).
In START, a randomized clinical trial that compared initiating ART in treatment-naive patients with CD4 counts >500 cells/mm3 versus waiting for a decrease to ≤350 cells/mm3 before initiation, there was a 53% reduction in serious illness and death in the early ART group Lundgren, et al. 2015. Data from NA-ACCORD, a large observational cohort study, showed that both morbidity and mortality were improved by initiation of ART in patients with CD4 cell counts in the high or even normal range Kitahata, et al. 2009. A significantly decreased risk of death was observed in patients who initiated therapy at CD4 counts >500 cells/mm3 compared with those who deferred therapy until CD4 count was <500 cells/mm3, as well as in the cohort who initiated ART in the 350 to 500 cells/mm3 range compared with those who deferred until CD4 count was <350 cells/mm3 Kitahata, et al. 2009. Although other cohort studies demonstrated only a minimal survival advantage Wright, et al. 2011 or no survival advantage among those starting ART at the highest CD4 cell counts, they did confirm the benefits of initiating ART at CD4 counts ≤500 cells/mm3 Young, et al. 2012; Cain, et al. 2011; CASCADE Collaboration 2011. Another study showed an approximately 33% reduction in the risk of death from end-stage liver disease, non-AIDS infections, and non-AIDS-defining cancers with each 100 cells/mm3 increase in CD4 count Marin, et al. 2009. A randomized study of early versus deferred therapy in patients with CD4 counts of 350 to 550 cells/mm3 showed no mortality benefit Cohen, et al. 2011; however, this study has significant limitations, most notably a relatively brief follow-up period.
Rationale for Rapid ART Initiation
This section is an excerpt from the NYSDOH AI guideline Rapid ART Initiation
| RECOMMENDATIONS |
Rationale for Rapid ART Initiation
|
The NYSDOH AI HIV Clinical Guidelines Program and the U.S. Department of Health and Human Services (DHHS) recommend initiation of ART for all patients with a confirmed HIV diagnosis, regardless of their CD4 cell count or viral load, for the benefit of the individual with HIV (reduced morbidity and mortality) Lundgren, et al. 2015; Zolopa, et al. 2009 and to reduce the risk of transmission to others Cohen, et al. 2016. Initiating ART during early HIV infection may improve immunologic recovery (CD4 T cell counts) and reduce the size of the HIV reservoir Massanella, et al. 2021; Jain, et al. 2013; evidence also shows that initiating ART at the time of diagnosis reduces treatment delays and improves retention in care and viral suppression at 12 months Ford, et al. 2018.
| KEY POINTS |
|
Reduced Treatment Delays and Loss to Follow-Up
Standard practice protocols for ART initiation have produced preventable delays, and the required wait for confirmatory HIV diagnostic and baseline laboratory test results (including resistance testing) along with required medical visits can unnecessarily delay the start of treatment by as long as 4 weeks. Problems in accessing insurance or waiting for activation of public benefits may also cause delays. It is estimated that in 2020, 82.4% of individuals diagnosed with HIV in the United States were linked to HIV medical care within 1 month of diagnosis CDC(b) 2022. Although not optimal, this reflects an increase since from 75.9% in 2016 CDC(b) 2022, before the first reports of rapid ART initiation. Individuals with HIV who are not linked to care are at risk of having sustained viral loads and ongoing HIV transmission.
Rapid ART initiation may reduce delays and improve viral suppression rates in people with HIV. Rapid or same-day ART initiation, which is preferable, or initiation within 3 days of a newly positive HIV test is the strategy endorsed by the World Health Organization WHO 2021 and is an essential component of the New York State Ending the Epidemic initiative. Mathematical modeling demonstrates that a test-and-treat strategy, with immediate initiation of ART and prevention approaches, could lead to elimination of new HIV infections Granich, et al. 2009.
Benefits for the Patient With HIV
Shorter time to viral suppression: Several observational and clinical trials have demonstrated the individual-level benefits of rapid ART initiation Ford, et al. 2018. An early pilot of this approach in San Francisco, California, demonstrated that patients initiating ART within 1 or 2 days had a shorter time (median, 1.8 months) to viral suppression (HIV RNA ≤200 copies/mL) than those offered the standard of care (4.3 months) or than historical controls (7.2 months) Pilcher, et al. 2017. A longer-term follow-up of 225 patients at the same center found that, of patients who had access to rapid initiation, 95.8% had achieved viral suppression at least once and 92.1% had achieved it at the last recorded visit Coffey, et al. 2019. These individual-level benefits have been replicated in other U.S. and international studies that demonstrated improved viral suppression with shortened time to ART initiation Mateo-Urdiales, et al. 2019; Mohammed, et al. 2019; Colasanti, et al. 2018; Koenig, et al. 2017; Rosen(b), et al. 2016. After implementing rapid ART initiation at a hospital clinic in Atlanta, Georgia, time to viral suppression fell from 77 days, before the intervention, to 57 days Lundgren, et al. 2015, and average time to ART initiation decreased from 21 to 7 days; both findings were statistically significant Colasanti, et al. 2018. After rollout of a city-wide rapid ART initiation program for people diagnosed with HIV in San Francisco, median time from first care visit to ART initiation decreased from 28 days to 1 day (by 96%) and median time from diagnosis to viral suppression decreased from 145 days to 76 days (by 46%) from 2013 to 2017 Bacon, et al. 2021.
Increased retention in care: Rapid ART initiation leads to improved retention in care Koenig, et al. 2017; Amanyire, et al. 2016; Rosen(b), et al. 2016. In the RapIT trial in South Africa, patients newly diagnosed with HIV were randomized to rapid ART initiation or standard of care Rosen(a), et al. 2016. The participants in the rapid initiation arm had higher rates of ART initiation at 90 days (97% vs. 72%) and higher rates of retention in care and viral suppression (HIV RNA ≤400 copies/mL) at 10 months (relative risk, 1.26 [1.05-1.50]). The average cost per patient to achieve viral suppression was lower in the intervention arm, demonstrating that this strategy of care may also be cost-effective Long, et al. 2017. Studies conducted in China, the United States, and South Africa support the cost-effectiveness of rapid ART initiation Benson, et al. 2020; Ford, et al. 2018; Wu, et al. 2015; Zulliger, et al. 2014. Rapid ART initiation is efficacious, safe, and highly acceptable, with few patients declining the offer of immediate ART Coffey, et al. 2019; Pilcher, et al. 2017.
Reduced HIV transmission: Modeling evidence suggests that rapid ART initiation may significantly reduce HIV transmission in the community, although this has been directly modeled only in the context of South Africa Granich, et al. 2009. In the United States, linkage to and retention in HIV care are significant gaps in the HIV care continuum, with an estimated 74.1% of individuals with HIV receiving any HIV care and 50.6% being retained in care during 2020 CDC(b) 2022. Models have translated these gaps in care to their effect on HIV transmission in the United States, demonstrating that between 43% and 49% of new HIV transmissions are attributable to individuals who have been diagnosed with HIV but are not receiving ART and have not been retained in care Li, et al. 2019; Skarbinski, et al. 2015. Because it is designed to help close this care gap, rapid ART initiation greatly reduces new HIV infections, hastening the achievement of HIV incidence reduction goals in New York State.
Rapid ART Initiation Is Safe
Preexisting resistance to currently recommended regimens for rapid initiation is rare. In the San Francisco study discussed previously Pilcher, et al. 2017, 89.7% of patients used integrase strand transfer inhibitor (INSTI)-containing regimens and 12.8% used protease inhibitor-containing regimens. The predominant INSTI-based regimen was dolutegravir plus emtricitabine/tenofovir disoproxil fumarate. The clinic did not have any cases of major resistance mutations to the prescribed ART regimen, and no regimen switches were made because of resistance. Two patients had their regimens changed because of rash, and in 10 cases, the regimen was simplified to a single-tablet regimen. Obtaining and following up on baseline laboratory testing is important, because some medical conditions, such as renal insufficiency, may require a change to a patient’s ART regimen.
Of 149 patients initiating ART through a program in New York City, only 1 required a regimen change because of subsequently detected resistance Pathela, et al. 2021.
Rapid ART initiation is safe. Most designated regimens for rapid ART initiation are the same regimens that are recommended for initial treatment in the existing NYSDOH, International Antiviral Society-USA, and DHHS guidelines. These regimens are well tolerated and effective, and the likelihood of drug resistance is low based on the current prevalence of drug resistance NYCDHMH 2021.
| Resources |
|
To identify or consult with an experienced HIV care provider in New York State, see the following: |
Protocol for Rapid ART Initiation
This section is an excerpt from the NYSDOH AI guideline Rapid ART Initiation
| RECOMMENDATIONS |
Protocol for Rapid ART Initiation
|
| SELECTED GOOD PRACTICE REMINDERS |
Protocol for Rapid ART Initiation
|
Figure 1: Protocol for Rapid ART Initiation
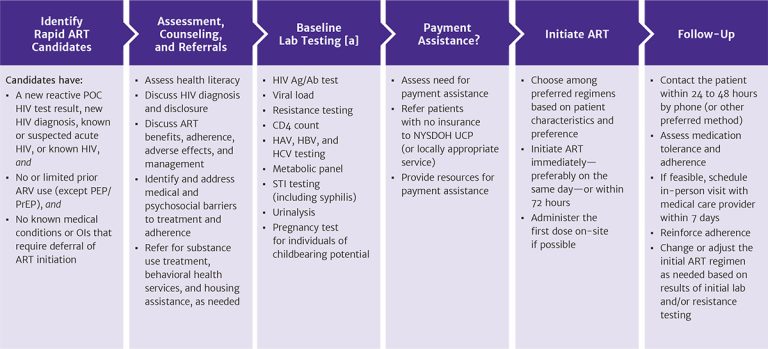
Abbreviations: Ag/Ab, antigen/antibody; ART, antiretroviral therapy; ARV, antiretroviral medication; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; NYSDOH UCP, New York State Department of Health Uninsured Care Programs; OI, opportunistic infection; PEP, post-exposure prophylaxis; POC, point-of-care; PrEP, pre-exposure prophylaxis; STI, sexually transmitted infection.
Note:
- ART can be started while awaiting laboratory test results.
Download figure: Protocol for Rapid ART Initiation
Reactive HIV Screening Test Result
When the result of a patient’s initial HIV point-of-care screening test is reactive, established practice is to obtain a blood specimen for diagnostic HIV testing because of the possibility of false-positive screening results. This is particularly important for individuals who are not at high risk of acquiring HIV. However, supplemental testing results may not be available for several days, introducing the risk that a patient will not return. The goal of the rapid ART initiation protocol is to assess whether a patient with a reactive HIV screening test result (or a confirmed HIV diagnosis) is also a candidate for same-day initiation of ART. If so, then the rapid ART initiation protocol is to provide counseling on HIV transmission and the benefits of ART, initiate ART that day or within 3 days, and link the patient expeditiously to HIV primary care. Thus, the protocol recommends immediate initiation of ART while awaiting confirmatory HIV test results.
Patients who are candidates for rapid ART initiation:
- Have a new reactive point-of-care HIV test result, a new HIV diagnosis (confirmed using the standard HIV laboratory testing algorithm), suspected acute HIV infection (HIV antibody negative and HIV RNA positive), or known HIV, and
- Are treatment naive or have limited prior use of antiretroviral medications (e.g., a patient who stopped first-line therapy for reasons other than regimen failure), excluding PEP or PrEP, as long as concern for acquired drug resistance is low (requires a case-by-case determination), and
- Have no medical conditions or opportunistic infections that require deferral of ART initiation, including suspected cryptococcal or TB meningitis or CMV retinitis
Patients with a new reactive HIV test result can be retested using a second point-of-care test from a manufacturer different from that of the first test to further minimize the possibility of a false-positive result. It is not necessary to retest with a second point-of-care test before providing ART, but given the possibility of a false-positive screening result, a laboratory-based confirmatory HIV test should always be performed to establish a diagnosis of HIV. If the confirmatory HIV test result is negative, ART can be discontinued.
| KEY POINT |
|
Counseling
A reactive HIV screening result should prompt a care provider to counsel the patient about the benefits and risks of ART and about HIV transmission risk, including the consensus that undetectable equals untransmittable (U=U). When patients initiate ART on the same day as their reactive HIV test result, the priorities for patient education and counseling include:
- Confirming the diagnosis of HIV
- Managing disclosure, if indicated
- Adhering to the ART regimen
- Ensuring the patient knows how to reach the care team to address any potential adverse effects of medications or other concerns
- Following through with clinic visits
- Assessing health literacy (see resources below)
- Navigating acquisition of and paying for medications required for lifelong therapy, including pharmacy selection, insurance requirements and restrictions, copays, and prescription refills
- Identifying and addressing psychosocial issues that may pose barriers to treatment
- Referring for substance use and behavioral health counseling if indicated
- Referring for housing assistance if indicated
| RESOURCES: HEALTH LITERACY |
|
Medical and Psychosocial Assessment
Medical assessment of a patient with a new reactive HIV test result should include history or signs or symptoms of opportunistic infection(s). ART should be delayed and appropriate medical management initiated if TB meningitis or cryptococcal meningitis are suspected (see below) WHO 2021, if cytomegalovirus retinitis is suspected, or if the patient has any evidence of advanced HIV disease on clinical exam.
To identify the potential for preexisting drug-resistant virus, the initial assessment should also include the patient’s history of PrEP and PEP use and previous ART use for people who are re-engaging in care Ford, et al. 2018. See Box 1, below.
| Box 1: Medical History Checklist |
When taking a medical history before rapid antiretroviral therapy (ART) initiation, ask about:
|
Deferral of ART initiation: If the patient understands the benefits of rapid initiation but declines ART, then initiation should be revisited as soon as possible. In some circumstances, such as in the rare case of suspected cryptococcal or TB meningitis, rapid ART is not recommended (see NYSDOH AI guideline Rapid ART Initiation > Special Considerations > Patients With Acute Opportunistic Infections). Patients who present with symptoms suggestive of CMV retinitis should be referred to an ophthalmologist for assessment and treatment. Patients who present with signs and symptoms suggestive of pulmonary or intracranial and ophthalmologic infections should receive further assessment before initiating ART on the same day as a reactive HIV screening test result.
ART initiation should be delayed in any person presenting with signs or symptoms suggestive of meningitis, including headache, nausea or vomiting, light sensitivity, and changes in mental status. Treatment of TB meningitis was investigated in a clinical trial in Vietnam in which immediate initiation of ART was compared with ART initiated 2 months after TB treatment Torok, et al. 2011. There were significantly more grade 4 adverse effects in individuals who initiated ART immediately than in those who delayed. Among patients with cryptococcal meningitis, early initiation of ART has been associated with adverse outcomes, including death Boulware, et al. 2014; therefore, it is recommended that ART be deferred until after the induction phase of treatment for cryptococcal meningitis has been completed (see U.S. Department of Health and Human Services Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV).
Cotreatment of HIV and pulmonary TB: It is clear that cotreatment of HIV and pulmonary TB improves survival. In the SAPIT trial in South Africa, there was a 56% relative reduction in mortality when ART was initiated within 4 weeks of TB treatment initiation, compared with when it was started after TB treatment was completed (hazard ratio, 0.44; 95% confidence interval, 0.25-0.79; P=.003), although symptoms of immune reconstitution inflammatory syndrome (IRIS) were greater in patients who started ART earlier Abdool Karim, et al. 2010. However, it is unclear whether ART initiation prior to initiation of pulmonary TB treatment is the best course of action. Care providers should weigh the benefits of rapid ART initiation against the potential drawbacks of pill burden, drug-drug interactions, and the risk of IRIS.
Baseline Laboratory and Resistance Testing
All patients with a reactive HIV test result should undergo the baseline laboratory testing listed in Box 2, below. For discussion of baseline testing, see the NYSDOH AI guideline Selecting an Initial ART Regimen > ART-Initiation Laboratory Testing. It is not necessary to wait for these test results before initiating ART.
| Box 2: Baseline Laboratory Testing Checklist |
|
All Recommendations
| ALL RECOMMENDATIONS: DIAGNOSIS AND MANAGEMENT OF ACUTE HIV INFECTION |
New York State HIV Testing Requirements
Presentation
When Acute HIV Infection Is Suspected
Diagnosis
ART Initiation
Partner Notification
Managing Acute HIV
Initiating ART
Benefits and Risks of ART
Rationale for Rapid ART Initiation
Protocol for Rapid ART Initiation
|
Shared Decision-Making
Download Printable PDF of Shared Decision-Making Statement
Date of current publication: August 8, 2023
Lead authors: Jessica Rodrigues, MS; Jessica M. Atrio, MD, MSc; and Johanna L. Gribble, MA
Writing group: Steven M. Fine, MD, PhD; Rona M. Vail, MD; Samuel T. Merrick, MD; Asa E. Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD
Committee: Medical Care Criteria Committee
Date of original publication: August 8, 2023
Rationale
Throughout its guidelines, the New York State Department of Health (NYSDOH) AIDS Institute (AI) Clinical Guidelines Program recommends “shared decision-making,” an individualized process central to patient-centered care. With shared decision-making, clinicians and patients engage in meaningful dialogue to arrive at an informed, collaborative decision about a patient’s health, care, and treatment planning. The approach to shared decision-making described here applies to recommendations included in all program guidelines. The included elements are drawn from a comprehensive review of multiple sources and similar attempts to define shared decision-making, including the Institute of Medicine’s original description [Institute of Medicine 2001]. For more information, a variety of informative resources and suggested readings are included at the end of the discussion.
Benefits
The benefits to patients that have been associated with a shared decision-making approach include:
- Decreased anxiety [Niburski, et al. 2020; Stalnikowicz and Brezis 2020]
- Increased trust in clinicians [Acree, et al. 2020; Groot, et al. 2020; Stalnikowicz and Brezis 2020]
- Improved engagement in preventive care [McNulty, et al. 2022; Scalia, et al. 2022; Bertakis and Azari 2011]
- Improved treatment adherence, clinical outcomes, and satisfaction with care [Crawford, et al. 2021; Bertakis and Azari 2011; Robinson, et al. 2008]
- Increased knowledge, confidence, empowerment, and self-efficacy [Chen, et al. 2021; Coronado-Vázquez, et al. 2020; Niburski, et al. 2020]
Approach
Collaborative care: Shared decision-making is an approach to healthcare delivery that respects a patient’s autonomy in responding to a clinician’s recommendations and facilitates dynamic, personalized, and collaborative care. Through this process, a clinician engages a patient in an open and respectful dialogue to elicit the patient’s knowledge, experience, healthcare goals, daily routine, lifestyle, support system, cultural and personal identity, and attitudes toward behavior, treatment, and risk. With this information and the clinician’s clinical expertise, the patient and clinician can collaborate to identify, evaluate, and choose from among available healthcare options [Coulter and Collins 2011]. This process emphasizes the importance of a patient’s values, preferences, needs, social context, and lived experience in evaluating the known benefits, risks, and limitations of a clinician’s recommendations for screening, prevention, treatment, and follow-up. As a result, shared decision-making also respects a patient’s autonomy, agency, and capacity in defining and managing their healthcare goals. Building a clinician-patient relationship rooted in shared decision-making can help clinicians engage in productive discussions with patients whose decisions may not align with optimal health outcomes. Fostering open and honest dialogue to understand a patient’s motivations while suspending judgment to reduce harm and explore alternatives is particularly vital when a patient chooses to engage in practices that may exacerbate or complicate health conditions [Halperin, et al. 2007].
Options: Implicit in the shared decision-making process is the recognition that the “right” healthcare decisions are those made by informed patients and clinicians working toward patient-centered and defined healthcare goals. When multiple options are available, shared decision-making encourages thoughtful discussion of the potential benefits and potential harms of all options, which may include doing nothing or waiting. This approach also acknowledges that efficacy may not be the most important factor in a patient’s preferences and choices [Sewell, et al. 2021].
Clinician awareness: The collaborative process of shared decision-making is enhanced by a clinician’s ability to demonstrate empathic interest in the patient, avoid stigmatizing language, employ cultural humility, recognize systemic barriers to equitable outcomes, and practice strategies of self-awareness and mitigation against implicit personal biases [Parish, et al. 2019].
Caveats: It is important for clinicians to recognize and be sensitive to the inherent power and influence they maintain throughout their interactions with patients. A clinician’s identity and community affiliations may influence their ability to navigate the shared decision-making process and develop a therapeutic alliance with the patient and may affect the treatment plan [KFF 2023; Greenwood, et al. 2020]. Furthermore, institutional policy and regional legislation, such as requirements for parental consent for gender-affirming care for transgender people or insurance coverage for sexual health care, may infringe upon a patient’s ability to access preventive- or treatment-related care [Sewell, et al. 2021].
Figure 1: Elements of Shared Decision-Making

Download figure: Elements of Shared Decision-Making
Health equity: Adapting a shared decision-making approach that supports diverse populations is necessary to achieve more equitable and inclusive health outcomes [Castaneda-Guarderas, et al. 2016]. For instance, clinicians may need to incorporate cultural- and community-specific considerations into discussions with women, gender-diverse individuals, and young people concerning their sexual behaviors, fertility intentions, and pregnancy or lactation status. Shared decision-making offers an opportunity to build trust among marginalized and disenfranchised communities by validating their symptoms, values, and lived experience. Furthermore, it can allow for improved consistency in patient screening and assessment of prevention options and treatment plans, which can reduce the influence of social constructs and implicit bias [Castaneda-Guarderas, et al. 2016].
Clinician bias has been associated with health disparities and can have profoundly negative effects [FitzGerald and Hurst 2017; Hall, et al. 2015]. It is often challenging for clinicians to recognize and set aside personal biases and to address biases with peers and colleagues. Consciously or unconsciously, negative or stigmatizing assumptions are often made about patient characteristics, such as race, ethnicity, gender, sexual orientation, mental health, and substance use [Avery, et al. 2019; van Boekel, et al. 2013; Livingston, et al. 2012]. With its emphasis on eliciting patient information, a shared decision-making approach encourages clinicians to inquire about patients’ lived experiences rather than making assumptions and to recognize the influence of that experience in healthcare decision-making.
Stigma: Stigma may prevent individuals from seeking or receiving treatment and harm reduction services [Tsai, et al. 2019]. Among people with HIV, stigma and medical mistrust remain significant barriers to healthcare utilization, HIV diagnosis, and medication adherence and can affect disease outcomes [Turan, et al. 2017; Chambers, et al. 2015], and stigma among clinicians against people who use substances has been well-documented [Stone, et al. 2021; Tsai, et al. 2019; van Boekel, et al. 2013]. Sexual and reproductive health, including strategies to prevent HIV transmission, acquisition, and progression, may be subject to stigma, bias, social influence, and violence.
| SHARED DECISION-MAKING IN HIV CARE |
|
Resources and Suggested Reading
In addition to the references cited below, the following resources and suggested reading may be useful to clinicians.
| RESOURCES |
References
Acree ME, McNulty M, Blocker O, et al. Shared decision-making around anal cancer screening among black bisexual and gay men in the USA. Cult Health Sex 2020;22(2):201-16. [PMID: 30931831]
Avery JD, Taylor KE, Kast KA, et al. Attitudes toward individuals with mental illness and substance use disorders among resident physicians. Prim Care Companion CNS Disord 2019;21(1):18m02382. [PMID: 30620451]
Bertakis KD, Azari R. Patient-centered care is associated with decreased health care utilization. J Am Board Fam Med 2011;24(3):229-39. [PMID: 21551394]
Castaneda-Guarderas A, Glassberg J, Grudzen CR, et al. Shared decision making with vulnerable populations in the emergency department. Acad Emerg Med 2016;23(12):1410-16. [PMID: 27860022]
Chambers LA, Rueda S, Baker DN, et al. Stigma, HIV and health: a qualitative synthesis. BMC Public Health 2015;15:848. [PMID: 26334626]
Chen CH, Kang YN, Chiu PY, et al. Effectiveness of shared decision-making intervention in patients with lumbar degenerative diseases: a randomized controlled trial. Patient Educ Couns 2021;104(10):2498-2504. [PMID: 33741234]
Coronado-Vázquez V, Canet-Fajas C, Delgado-Marroquín MT, et al. Interventions to facilitate shared decision-making using decision aids with patients in primary health care: a systematic review. Medicine (Baltimore) 2020;99(32):e21389. [PMID: 32769870]
Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf
Crawford J, Petrie K, Harvey SB. Shared decision-making and the implementation of treatment recommendations for depression. Patient Educ Couns 2021;104(8):2119-21. [PMID: 33563500]
FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics 2017;18(1):19. [PMID: 28249596]
Greenwood BN, Hardeman RR, Huang L, et al. Physician-patient racial concordance and disparities in birthing mortality for newborns. Proc Natl Acad Sci U S A 2020;117(35):21194-21200. [PMID: 32817561]
Groot G, Waldron T, Barreno L, et al. Trust and world view in shared decision making with indigenous patients: a realist synthesis. J Eval Clin Pract 2020;26(2):503-14. [PMID: 31750600]
Hall WJ, Chapman MV, Lee KM, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60-76. [PMID: 26469668]
Halperin B, Melnychuk R, Downie J, et al. When is it permissible to dismiss a family who refuses vaccines? Legal, ethical and public health perspectives. Paediatr Child Health 2007;12(10):843-45. [PMID: 19043497]
Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001. https://www.ncbi.nlm.nih.gov/books/NBK222274/
KFF. Key data on health and health care by race and ethnicity. 2023 Mar 15. https://www.kff.org/racial-equity-and-health-policy/report/key-data-on-health-and-health-care-by-race-and-ethnicity/ [accessed 2023 May 19]
Livingston JD, Milne T, Fang ML, et al. The effectiveness of interventions for reducing stigma related to substance use disorders: a systematic review. Addiction 2012;107(1):39-50. [PMID: 21815959]
McNulty MC, Acree ME, Kerman J, et al. Shared decision making for HIV pre-exposure prophylaxis (PrEP) with black transgender women. Cult Health Sex 2022;24(8):1033-46. [PMID: 33983866]
Niburski K, Guadagno E, Abbasgholizadeh-Rahimi S, et al. Shared decision making in surgery: a meta-analysis of existing literature. Patient 2020;13(6):667-81. [PMID: 32880820]
Parish SJ, Hahn SR, Goldstein SW, et al. The International Society for the Study of Women’s Sexual Health process of care for the identification of sexual concerns and problems in women. Mayo Clin Proc 2019;94(5):842-56. [PMID: 30954288]
Robinson JH, Callister LC, Berry JA, et al. Patient-centered care and adherence: definitions and applications to improve outcomes. J Am Acad Nurse Pract 2008;20(12):600-607. [PMID: 19120591]
Scalia P, Durand MA, Elwyn G. Shared decision-making interventions: an overview and a meta-analysis of their impact on vaccine uptake. J Intern Med 2022;291(4):408-25. [PMID: 34700363]
Sewell WC, Solleveld P, Seidman D, et al. Patient-led decision-making for HIV preexposure prophylaxis. Curr HIV/AIDS Rep 2021;18(1):48-56. [PMID: 33417201]
Stalnikowicz R, Brezis M. Meaningful shared decision-making: complex process demanding cognitive and emotional skills. J Eval Clin Pract 2020;26(2):431-38. [PMID: 31989727]
Stone EM, Kennedy-Hendricks A, Barry CL, et al. The role of stigma in U.S. primary care physicians’ treatment of opioid use disorder. Drug Alcohol Depend 2021;221:108627. [PMID: 33621805]
Tsai AC, Kiang MV, Barnett ML, et al. Stigma as a fundamental hindrance to the United States opioid overdose crisis response. PLoS Med 2019;16(11):e1002969. [PMID: 31770387]
Turan B, Budhwani H, Fazeli PL, et al. How does stigma affect people living with HIV? The mediating roles of internalized and anticipated HIV stigma in the effects of perceived community stigma on health and psychosocial outcomes. AIDS Behav 2017;21(1):283-91. [PMID: 27272742]
van Boekel LC, Brouwers EP, van Weeghel J, et al. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend 2013;131(1-2):23-35. [PMID: 23490450]
References
Abdool Karim S. S., Naidoo K., Grobler A., et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 2010;362(8):697-706. [PMID: 20181971]
Amanyire G., Semitala F. C., Namusobya J., et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV 2016;3(11):e539-48. [PMID: 27658873]
Ananworanich J., Schuetz A., Vandergeeten C., et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS One 2012;7(3):e33948. [PMID: 22479485]
Apoola A., Ahmad S., Radcliffe K. Primary HIV infection. Int J STD AIDS 2002;13(2):71-78. [PMID: 11839160]
Bacon O., Chin J., Cohen S. E., et al. Decreased time from human immunodeficiency virus diagnosis to care, antiretroviral therapy initiation, and virologic suppression during the citywide RAPID Initiative in San Francisco. Clin Infect Dis 2021;73(1):e122-28. [PMID: 32449916]
Bavinton B. R., Pinto A. N., Phanuphak N., et al. Viral suppression and HIV transmission in serodiscordant male couples: an international, prospective, observational, cohort study. Lancet HIV 2018;5(8):e438-47. [PMID: 30025681]
Bellan S. E., Dushoff J., Galvani A. P., et al. Reassessment of HIV-1 acute phase infectivity: accounting for heterogeneity and study design with simulated cohorts. PLoS Med 2015;12(3):e1001801. [PMID: 25781323]
Benson C., Emond B., Lefebvre P., et al. Rapid initiation of antiretroviral therapy following diagnosis of human immunodeficiency virus among patients with commercial insurance coverage. J Manag Care Spec Pharm 2020;26(2):129-41. [PMID: 31747358]
Boulware D. R., Meya D. B., Muzoora C., et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014;370(26):2487-98. [PMID: 24963568]
Bourgi(a) K., Jenkins C. A., Rebeiro P. F., et al. Weight gain among treatment-naive persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc 2020;23(4):e25484. [PMID: 32294337]
Bourgi(b) K., Rebeiro P. F., Turner M., et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis 2020;70(7):1267-74. [PMID: 31100116]
Bruyand M., Thiebaut R., Lawson-Ayayi S., et al. Role of uncontrolled HIV RNA level and immunodeficiency in the occurrence of malignancy in HIV-infected patients during the combination antiretroviral therapy era: Agence Nationale de Recherche sur le Sida (ANRS) CO3 Aquitaine Cohort. Clin Infect Dis 2009;49(7):1109-16. [PMID: 19705973]
Buzon M., Siess K., Sone A. Treatment of early HIV infection reduces viral reservoir to levels found in elite controllers. Abstract 151. CROI; 2012 Mar 5-8.
Cain L. E., Logan R., Robins J. M., et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med 2011;154(8):509-15. [PMID: 21502648]
CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med 2011;171(17):1560-69. [PMID: 21949165]
CDC(a). HIV surveillance report, 2020. 2022 Aug 26. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [accessed 2023 Feb 8]
CDC(b). Monitoring selected national HIV prevention and care objectives by using HIV surveillance data United States and 6 dependent areas, 2020. 2022 Aug. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-27-3.pdf [accessed 2023 Feb 8]
Chin T., Hicks C., Samsa G., et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS 2013;27(7):392-97. [PMID: 23802143]
Coffey S., Bacchetti P., Sachdev D., et al. RAPID antiretroviral therapy: high virologic suppression rates with immediate antiretroviral therapy initiation in a vulnerable urban clinic population. AIDS 2019;33(5):825-32. [PMID: 30882490]
Cohen M. S., Chen Y. Q., McCauley M., et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med 2016;375(9):830-39. [PMID: 27424812]
Cohen M. S., Chen Y. Q., McCauley M., et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493-505. [PMID: 21767103]
Colasanti J., Sumitani J., Mehta C. C., et al. Implementation of a rapid entry program decreases time to viral suppression among vulnerable persons living with HIV in the southern United States. Open Forum Infect Dis 2018;5(6):ofy104. [PMID: 29992172]
Colfax G. N., Buchbinder S. P., Cornelisse P. G., et al. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS 2002;16(11):1529-35. [PMID: 12131191]
Connor E. M., Sperling R. S., Gelber R., et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med 1994;331(18):1173-80. [PMID: 7935654]
DHHS. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2019 Dec 18. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/acute-and-recent-early-hiv-infection [accessed 2021 Jun 29]
El-Sadr W. M., Lundgren J., Neaton J. D., et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006;355(22):2283-96. [PMID: 17135583]
Ellis R. J., Badiee J., Vaida F., et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS 2011;25(14):1747-51. [PMID: 21750419]
Fonner V. A., Denison J., Kennedy C. E., et al. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev 2012;9(9):CD001224. [PMID: 22972050]
Ford N., Migone C., Calmy A., et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018;32(1):17-23. [PMID: 29112073]
Friis-Moller N., Thiebaut R., Reiss P., et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010;17(5):491-501. [PMID: 20543702]
Garvey L., Surendrakumar V., Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials 2011;12(6):333-38. [PMID: 22189152]
Golden M. R., Hogben M., Potterat J. J., et al. HIV partner notification in the United States: a national survey of program coverage and outcomes. Sex Transm Dis 2004;31(12):709-12. [PMID: 15608584]
Granich R. M., Gilks C. F., Dye C., et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009;373(9657):48-57. [PMID: 19038438]
Guay L. A., Musoke P., Fleming T., et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 1999;354(9181):795-802. [PMID: 10485720]
Guiguet M., Boue F., Cadranel J., et al. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 2009;10(12):1152-59. [PMID: 19818686]
Hare C. B., Pappalardo B. L., Busch M. P., et al. Seroreversion in subjects receiving antiretroviral therapy during acute/early HIV infection. Clin Infect Dis 2006;42(5):700-708. [PMID: 16447118]
Hecht F. M., Busch M. P., Rawal B., et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS 2002;16(8):1119-29. [PMID: 12004270]
Hileman C. O., Funderburg N. T. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep 2017;14(3):93-100. [PMID: 28434169]
Ho J. E., Deeks S. G., Hecht F. M., et al. Initiation of antiretroviral therapy at higher nadir CD4+ T-cell counts is associated with reduced arterial stiffness in HIV-infected individuals. AIDS 2010;24(12):1897-1905. [PMID: 20543654]
Ho J. E., Scherzer R., Hecht F. M., et al. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS 2012;26(9):1115-20. [PMID: 22382147]
Hocqueloux L., Prazuck T., Avettand-Fenoel V., et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS 2010;24(10):1598-1601. [PMID: 20549847]
Hollingsworth T. D., Anderson R. M., Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis 2008;198(5):687-93. [PMID: 18662132]
Hollingsworth T. D., Pilcher C. D., Hecht F. M., et al. High transmissibility during early HIV infection among men who have sex with men-San Francisco, California. J Infect Dis 2015;211(11):1757-60. [PMID: 25542958]
Hoy J. F., Grund B., Roediger M., et al. Immediate initiation of antiretroviral therapy for HIV infection accelerates bone loss relative to deferring therapy: findings from the START Bone Mineral Density Substudy, a randomized trial. J Bone Miner Res 2017;32(9):1945-55. [PMID: 28650589]
Jain V., Hartogensis W., Bacchetti P., et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis 2013;208(8):1202-11. [PMID: 23852127]
Kassutto S., Johnston M. N., Rosenberg E. S. Incomplete HIV type 1 antibody evolution and seroreversion in acutely infected individuals treated with early antiretroviral therapy. Clin Infect Dis 2005;40(6):868-73. [PMID: 15736021]
Kitahata M. M., Gange S. J., Abraham A. G., et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009;360(18):1815-26. [PMID: 19339714]
Koegl C., Wolf E., Hanhoff N., et al. Treatment during primary HIV infection does not lower viral set point but improves CD4 lymphocytes in an observational cohort. Eur J Med Res 2009;14(7):277-83. [PMID: 19661009]
Koenig S. P., Dorvil N., Dévieux J. G., et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: a randomized unblinded trial. PLoS Med 2017;14(7):e1002357. [PMID: 28742880]
Kroon Edmb, Phanuphak N., Shattock A. J., et al. Acute HIV infection detection and immediate treatment estimated to reduce transmission by 89% among men who have sex with men in Bangkok. J Int AIDS Soc 2017;20(1):21708. [PMID: 28691441]
Lafeuillade A., Hittinger G., Lambry V. Long-term control of HIV reservoir after a 2-year ART course at acute infection. Abstract 358. CROI; 2012 Mar 5-8.
Le T., Wright E. J., Smith D. M., et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013;368(3):218-30. [PMID: 23323898]
Li X., Margolick J. B., Jamieson B. D., et al. CD4+ T-cell counts and plasma HIV-1 RNA levels beyond 5 years of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2011;57(5):421-28. [PMID: 21602699]
Li Z., Purcell D. W., Sansom S. L., et al. Vital signs: HIV transmission along the continuum of care - United States, 2016. MMWR Morb Mortal Wkly Rep 2019;68(11):267-72. [PMID: 30897075]
Lichtenstein K. A., Armon C., Buchacz K., et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010;51(4):435-47. [PMID: 20597691]
Long L. C., Maskew M., Brennan A. T., et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: a cost-effectiveness analysis of the rapid initiation of treatment randomized controlled trial. AIDS 2017;31(11):1611-19. [PMID: 28463879]
Lundgren J. D., Babiker A. G., Gordin F., et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373(9):795-807. [PMID: 26192873]
Lundgren J. D., Babiker A. G., Sharma S., et al. Long-term benefits from early antiretroviral therapy initiation in HIV infection. NEJM Evid 2023;2(3):10. [PMID: 37213438]
Ma Z. M., Stone M., Piatak M., et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol 2009;83(7):3288-97. [PMID: 19129448]
Manabe Y. C., Campbell J. D., Sydnor E., et al. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr 2007;46(4):456-62. [PMID: 18077835]
Margolick J. B., Apuzzo L., Singer J., et al. A randomized trial of time-limited antiretroviral therapy in acute/early HIV infection. PLoS One 2015;10(11):e0143259. [PMID: 26600459]
Marin B., Thiebaut R., Bucher H. C., et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS 2009;23(13):1743-53. [PMID: 19571723]
Massanella M., Bender Ignacio R. A., Lama J. R., et al. Long-term effects of early antiretroviral initiation on HIV reservoir markers: a longitudinal analysis of the MERLIN clinical study. Lancet Microbe 2021;2(5):e198-209. [PMID: 35544209]
Mateo-Urdiales A., Johnson S., Smith R., et al. Rapid initiation of antiretroviral therapy for people living with HIV. Cochrane Database Syst Rev 2019;6:CD012962. [PMID: 31206168]
Metsch L. R., Pereyra M., Messinger S., et al. HIV transmission risk behaviors among HIV-infected persons who are successfully linked to care. Clin Infect Dis 2008;47(4):577-84. [PMID: 18624629]
Mohammed D. Y., Martin E., Brewer R., et al. Same-day medical visit increases viral suppression, Peter Ho Memorial Clinic, 2014-2015 and 2016-2017. J Assoc Nurses AIDS Care 2019;30(3):292-300. [PMID: 30676360]
Monteiro N., Branco M., Peres S., et al. The impact of tenofovir disoproxil fumarate on kidney function: four-year data from the HIV-infected outpatient cohort. J Int AIDS Soc 2014;17(4 Suppl 3):19565. [PMID: 25394072]
Nakao J. H., Wiener D. E., Newman D. H., et al. Falling through the cracks? Missed opportunities for earlier HIV diagnosis in a New York City Hospital. Int J STD AIDS 2014;25(12):887-93. [PMID: 24535693]
NYCDHMH. HIV surveillance annual report, 2020. 2021 Dec. https://www.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2020.pdf [accessed 2023 Feb 8]
NYSDOH. Dear colleague letter. 2017 Sep. https://www.health.ny.gov/diseases/aids/ending_the_epidemic/docs/september_physician_letter.pdf [accessed 2018 Aug 21]
O'Connor J., Vjecha M. J., Phillips A. N., et al. Effect of immediate initiation of antiretroviral therapy on risk of severe bacterial infections in HIV-positive people with CD4 cell counts of more than 500 cells per muL: secondary outcome results from a randomised controlled trial. Lancet HIV 2017;4(3):e105-12. [PMID: 28063815]
Palella F. J., Hou Q., Li J., et al. Weight gain and metabolic effects in persons With HIV who switch to ART regimens containing integrase inhibitors or tenofovir alafenamide. J Acquir Immune Defic Syndr 2023;92(1):67-75. [PMID: 36150045]
Patel P., Klausner J. D., Bacon O. M., et al. Detection of acute HIV infections in high-risk patients in California. J Acquir Immune Defic Syndr 2006;42(1):75-79. [PMID: 16763493]
Pathela P., Jamison K., Braunstein S. L., et al. Initiating antiretroviral treatment for newly diagnosed HIV patients in sexual health clinics greatly improves timeliness of viral suppression. AIDS 2021;35(11):1805-12. [PMID: 33973874]
Patterson K. B., Leone P. A., Fiscus S. A., et al. Frequent detection of acute HIV infection in pregnant women. AIDS 2007;21(17):2303-8. [PMID: 18090278]
Phanuphak N., Teeratakulpisarn N., van Griensven F., et al. Anogenital HIV RNA in Thai men who have sex with men in Bangkok during acute HIV infection and after randomization to standard vs. intensified antiretroviral regimens. J Int AIDS Soc 2015;18(1):19470. [PMID: 25956171]
Pilcher C. D., Ospina-Norvell C., Dasgupta A., et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr 2017;74(1):44-51. [PMID: 27434707]
Pilcher C. D., Tien H. C., Eron J. J., et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis 2004;189(10):1785-92. [PMID: 15122514]
Pinkerton S. D. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS Behav 2008;12(5):677-84. [PMID: 18064559]
Pires A., Hardy G., Gazzard B., et al. Initiation of antiretroviral therapy during recent HIV-1 infection results in lower residual viral reservoirs. J Acquir Immune Defic Syndr 2004;36(3):783-90. [PMID: 15213561]
Prevention Access Campaign. Prevention Access Campaign consensus statement: risk of sexual transmission of HIV from a person living with HIV who has an undetectable viral load. 2018 Jan 28. https://www.preventionaccess.org/consensus [accessed 2018 Aug 21]
Quinn T. C., Wawer M. J., Sewankambo N., et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000;342(13):921-29. [PMID: 10738050]
Robb M. L., Eller L. A., Kibuuka H., et al. Prospective study of acute HIV-1 infection in adults in East Africa and Thailand. N Engl J Med 2016;374(22):2120-30. [PMID: 27192360]
Rodger A. J., Cambiano V., Bruun T., et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 2019;393(10189):2428-38. [PMID: 31056293]
Rodger A. J., Cambiano V., Bruun T., et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner Is using suppressive antiretroviral therapy. JAMA 2016;316(2):171-81. [PMID: 27404185]
Rosen(a) S., Maskew M., Fox M. P., et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: the RapIT randomized controlled trial. PLoS Med 2016;13(5):e1002015. [PMID: 27163694]
Rosen(b) S., Maskew M., Fox M. P., et al. Correction: Initiating antiretroviral therapy for HIV at a patient's first clinic visit: the RapIT randomized controlled trial. PLoS Med 2016;13(6):e1002050. [PMID: 27258028]
Rutstein S. E., Ananworanich J., Fidler S., et al. Clinical and public health implications of acute and early HIV detection and treatment: a scoping review. J Int AIDS Soc 2017;20(1):21579. [PMID: 28691435]
Sabin C. A., Smith C. J., d'Arminio Monforte A., et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008;22(12):1463-73. [PMID: 18614870]
Saez-Cirion A., Bacchus C., Hocqueloux L., et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 2013;9(3):e1003211. [PMID: 23516360]
Scherrer A. U., von Wyl V., Yang W. L., et al. Emergence of acquired HIV-1 drug resistance almost stopped in Switzerland: a 15-year prospective cohort analysis. Clin Infect Dis 2016;62(10):1310-17. [PMID: 26962075]
Severe P., Juste M. A., Ambroise A., et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med 2010;363(3):257-65. [PMID: 20647201]
Siddiqi A. E., Hall H. I., Hu X., et al. Population-based estimates of life expectancy after HIV diagnosis: United States 2008-2011. J Acquir Immune Defic Syndr 2016;72(2):230-36. [PMID: 26890283]
Sigel K., Wisnivesky J., Gordon K., et al. HIV as an independent risk factor for incident lung cancer. AIDS 2012;26(8):1017-25. [PMID: 22382152]
Silverberg M. J., Chao C., Leyden W. A., et al. HIV infection, immunodeficiency, viral replication, and the risk of cancer. Cancer Epidemiol Biomarkers Prev 2011;20(12):2551-59. [PMID: 22109347]
Skarbinski J., Rosenberg E., Paz-Bailey G., et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015;175(4):588-96. [PMID: 25706928]
Stekler J. D., Violette L. R., Niemann L. A., et al. Seroconversion, seroreversion, and serowaffling among participants initiating antiretroviral therapy in Project DETECT. Int J STD AIDS 2023;34(6):385-94. [PMID: 36703607]
Steward W. T., Remien R. H., Higgins J. A., et al. Behavior change following diagnosis with acute/early HIV infection-a move to serosorting with other HIV-infected individuals. The NIMH Multisite Acute HIV Infection Study: III. AIDS Behav 2009;13(6):1054-60. [PMID: 19504178]
Streeck H., Jessen H., Alter G., et al. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis 2006;194(6):734-39. [PMID: 16941338]
Torok M. E., Yen N. T., Chau T. T., et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis. Clin Infect Dis 2011;52(11):1374-83. [PMID: 21596680]
Tozzi V., Balestra P., Bellagamba R., et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr 2007;45(2):174-82. [PMID: 17356465]
van Lelyveld S. F., Gras L., Kesselring A., et al. Long-term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS 2012;26(4):465-74. [PMID: 22112603]
Verburgh M. L., Wit F. W., Boyd A., et al. One in 10 virally suppressed persons with HIV in The Netherlands experiences >/=10% weight gain after switching to tenofovir alafenamide and/or integrase strand transfer inhibitor. Open Forum Infect Dis 2022;9(7):ofac291. [PMID: 35873291]
WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. 2021 16 Jul. https://www.who.int/publications/i/item/9789240031593 [accessed 2023 Feb 8]
Winston A., Puls R., Kerr S. J., et al. Dynamics of cognitive change in HIV-infected individuals commencing three different initial antiretroviral regimens: a randomized, controlled study. HIV Med 2012;13(4):245-51. [PMID: 22151608]
Wright S. T., Carr A., Woolley I., et al. CD4 cell responses to combination antiretroviral therapy in patients starting therapy at high CD4 cell counts. J Acquir Immune Defic Syndr 2011;58(1):72-79. [PMID: 21654498]
Wu Z., Zhao Y., Ge X., et al. Simplified HIV testing and treatment in China: analysis of mortality rates before and after a structural intervention. PLoS Med 2015;12(9):e1001874. [PMID: 26348214]
Xia Q., Maduro G. A., Li W., et al. Life expectancy among people with HIV in New York City, 2009-2018. J Acquir Immune Defic Syndr 2022;91(5):434-38. [PMID: 36084201]
Young J., Psichogiou M., Meyer L., et al. CD4 cell count and the risk of AIDS or death in HIV-Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med 2012;9(3):e1001194. [PMID: 22448150]
Zolopa A., Andersen J., Powderly W., et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One 2009;4(5):e5575. [PMID: 19440326]
Zulliger R., Black S., Holtgrave D. R., et al. Cost-effectiveness of a package of interventions for expedited antiretroviral therapy initiation during pregnancy in Cape Town, South Africa. AIDS Behav 2014;18(4):697-705. [PMID: 24122044]
Updates, Authorship, and Related Guidelines
| Updates, Authorship, and Related Guidelines | |
| Date of original publication | August 24, 2018 |
| Date of current publication | July 19, 2021 |
| Highlights of changes, additions, and updates in the July 19, 2021 edition |
|
| Intended users | Clinicians in New York State who provide ambulatory, inpatient, and emergency medical care for adults ≥18 years old who present with signs or symptoms of acute HIV infection or report an exposure within the past 4 weeks |
| Lead author |
Ethan A. Cowan, MD, MS |
| Writing group |
Joseph P. McGowan, MD, FACP, FIDSA; Steven M. Fine, MD, PhD; Rona Vail, MD; Samuel T. Merrick, MD; Asa Radix, MD, MPH, PhD; Christopher J. Hoffmann, MD, MPH; Charles J. Gonzalez, MD |
| Author and writing group conflict of interest disclosures | There are no author or writing group conflict of interest disclosures. |
| Committee | |
| Developer and funder |
New York State Department of Health AIDS Institute (NYSDOH AI) |
| Development process |
See Guideline Development and Recommendation Ratings Scheme, below. |
| Related NYSDOH AI guidelines | |
Guideline Development and Recommendation Ratings
| Guideline Development: New York State Department of Health AIDS Institute Clinical Guidelines Program | |
| Program manager | Clinical Guidelines Program, Johns Hopkins University School of Medicine, Division of Infectious Diseases. See Program Leadership and Staff. |
| Mission | To produce and disseminate evidence-based, state-of-the-art clinical practice guidelines that establish uniform standards of care for practitioners who provide prevention or treatment of HIV, viral hepatitis, other sexually transmitted infections, and substance use disorders for adults throughout New York State in the wide array of settings in which those services are delivered. |
| Expert committees | The NYSDOH AI Medical Director invites and appoints committees of clinical and public health experts from throughout New York State to ensure that the guidelines are practical, immediately applicable, and meet the needs of care providers and stakeholders in all major regions of New York State, all relevant clinical practice settings, key New York State agencies, and community service organizations. |
| Committee structure |
|
| Disclosure and management of conflicts of interest |
|
| Evidence collection and review |
|
| Recommendation development |
|
| Review and approval process |
|
| External reviews |
|
| Update process |
|
| Recommendation Ratings Scheme | |||
| Strength | Quality of Evidence | ||
| Rating | Definition | Rating | Definition |
| A | Strong | 1 | Based on published results of at least 1 randomized clinical trial with clinical outcomes or validated laboratory endpoints. |
| B | Moderate | * | Based on either a self-evident conclusion; conclusive, published, in vitro data; or well-established practice that cannot be tested because ethics would preclude a clinical trial. |
| C | Optional | 2 | Based on published results of at least 1 well-designed, nonrandomized clinical trial or observational cohort study with long-term clinical outcomes. |
| 2† | Extrapolated from published results of well-designed studies (including nonrandomized clinical trials) conducted in populations other than those specifically addressed by a recommendation. The source(s) of the extrapolated evidence and the rationale for the extrapolation are provided in the guideline text. One example would be results of studies conducted predominantly in a subpopulation (e.g., one gender) that the committee determines to be generalizable to the population under consideration in the guideline. | ||
| 3 | Based on committee expert opinion, with rationale provided in the guideline text. | ||
Last updated on January 18, 2024

